Diving Deep into Pressure: Unveiling Nature's Hidden Strength
Prepare to uncover the invisible force that shapes our world and drives remarkable phenomena. Step into the captivating realm of pressure, where squeezing, pushing, and intense energy collide. From the depths of the ocean to the marvels of engineering, we'll embark on a journey to unravel the secrets and applications of this powerful yet often overlooked concept. Get ready to be amazed by the extraordinary force of pressure!
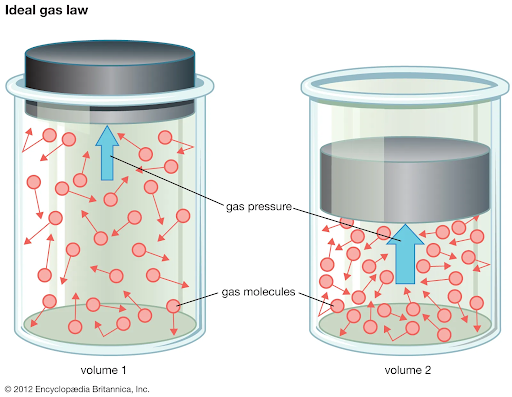
Fig 1. Gas pressure
What does pressure mean?
Pressure is a physical quantity that measures the amount of force applied per unit area. It is defined as the force exerted perpendicular to the surface of an object divided by the area over which the force is distributed. In simpler terms, pressure is a measure of how much "push" or "squeeze" is being applied to an object or a fluid.
The SI (International System of Units) unit for pressure is the pascal ( Pa), which is equal to one newton per square meter (N/m²). However, other units such as pounds per square inch (psi), bar, and atmospheres (atm) are also commonly used.
Pressure can be exerted by solids, liquids, and gases. When a force is applied to a solid object, the pressure is calculated by dividing the force by the area over which the force is distributed. In the case of a fluid (liquid or gas), pressure arises due to the random motion and collisions of its molecules or atoms with the walls of the container or other objects.
Pressure has numerous applications in various fields, including physics, engineering, meteorology, and many branches of science and technology. It plays a crucial role in understanding fluid dynamics, measuring atmospheric conditions, designing hydraulic systems, calculating mechanical stress, and much more.
What are the two factors affecting pressure?
The pressure of a fluid or on a surface is influenced by two primary factors:
- Force: Force is one of the key factors that affect pressure. The magnitude of the force applied perpendicular to a surface determines the amount of pressure exerted. A larger force applied over a given area results in higher pressure, while a smaller force leads to lower pressure. For example, pushing against a wall with greater force will increase the pressure exerted on the wall.
- Area: The area over which the force is distributed is another crucial factor influencing pressure. Increasing the surface area over which a force is applied decreases the pressure while decreasing the surface area leads to higher pressure. This can be understood using the formula for pressure: P = F / A, where P represents pressure, F represents force, and A represents area. As the area increases while the force remains constant, the pressure decreases, and vice versa.
To summarize, the pressure is directly proportional to the force applied and inversely proportional to the area over which the force is distributed. These two factors play a significant role in determining the intensity or strength of the pressure exerted on a surface or within a fluid.

Fig 2. Pressure definition
What does pressure mean in the industry?
In the context of the industry, pressure refers to the force or stress exerted on a system or equipment within a manufacturing or production environment. It is a critical parameter that is closely monitored and controlled in many industrial processes.
Here are a few examples of how pressure is relevant in different industrial sectors:
- Manufacturing: In manufacturing processes, pressure may be applied to shape, mold, or compress materials. For instance, in metalworking, hydraulic or pneumatic presses apply pressure to shape or form metal sheets. In plastic injection molding, high-pressure systems are used to inject molten plastic into molds.
- Oil and Gas: Pressure plays a crucial role in the extraction, transportation, and processing of oil and gas. In drilling operations, pressure is monitored to ensure safe and efficient extraction from wells. Pipelines and storage tanks are designed to handle specific pressures and pressure control devices are employed to maintain operational safety.
- Chemical Industry: Many chemical processes involve pressurized systems. Pressure vessels, reactors, and distillation columns are examples of equipment that operate under specific pressures to facilitate chemical reactions, separations, and purification processes.
- HVAC and Refrigeration: Pressure is significant in heating, ventilation, air conditioning (HVAC), and refrigeration systems. Compressors, which are central components in these systems, increase the pressure of refrigerants to facilitate cooling or heating. Pressure measurements are essential for system efficiency, safety, and troubleshooting.
- Power Generation: Power plants, whether nuclear, thermal, or renewable, utilize pressure in various ways. Steam turbines are driven by high-pressure steam, and boilers are designed to withstand specific pressures. In hydroelectric power plants, pressure is created by water flow to generate electricity.
In all these industrial applications, pressure monitoring, regulation, and safety precautions are critical to ensure equipment integrity, operational efficiency, and worker safety.

Fig 3. Different types of pressure
As you may reckon, in the industrial world, pressure is a vital parameter that is measured and controlled using specialized instruments like pressure sensors. These sensors provide accurate and real-time data essential for maintaining equipment integrity, operational efficiency, and worker safety. To explore a range of pressure sensors suitable for various applications, check out our pressure sensor category:
What is pressure in an electronic?
In electronics, pressure typically refers to a specialized sensor known as a pressure sensor or pressure transducer. A pressure sensor is an electronic device that measures the pressure exerted on it and converts it into an electrical signal that can be measured and processed by other electronic components.
Pressure sensors are commonly used in various electronic systems and devices for different purposes. Here are a few examples:
- Barometric Pressure Sensor: Barometric pressure sensors are used to measure atmospheric pressure. They are employed in weather monitoring equipment, altimeters, and GPS devices to determine altitude and provide accurate location information.
- Industrial Process Control: In industrial settings, pressure sensors are utilized to monitor and control pressure levels in manufacturing processes. They ensure that the pressure within systems, pipelines, or vessels is maintained within the desired range for safe and efficient operation.
- Automotive Applications: Pressure sensors play a vital role in automotive systems. They are used in tire pressure monitoring systems (TPMS) to measure the air pressure in tires and alert the driver in case of low pressure. Additionally, they are employed in engine management systems to monitor intake manifold pressure, fuel pressure, and other parameters for optimal engine performance.
- Medical Devices: Pressure sensors are integral to various medical devices and equipment. They are used in blood pressure monitors, respiratory devices, infusion pumps, and other medical instruments to measure and regulate pressure levels accurately.
- Consumer Electronics: Pressure sensors are also found in consumer electronics devices. For example, smartphones may include barometric pressure sensors to provide altitude data for fitness tracking or to enhance location-based services.
The actual implementation and technology of pressure sensors can vary. Some common types include piezoresistive sensors, capacitive sensors, and optical sensors. Each type utilizes different principles to convert pressure into an electrical signal.
Overall, pressure sensors in electronics enable the measurement, monitoring, and control of pressure parameters, enhancing the functionality and safety of various systems and devices.
What is the pressure formula SI?
In the SI (International System of Units), the formula for pressure is derived from the fundamental units of force and area. The SI unit for pressure is the pascal (Pa), which is defined as one newton per square meter (N/m²).
The formula for pressure (P) in the SI system is:
P = F / A
Where: P is the pressure in pascals (Pa), F is the force applied perpendicular to the surface in newtons (N), and A is the area over which the force is applied in square meters (m²).
This formula states that pressure is equal to the force divided by the area on which the force is distributed. Essentially, it measures the amount of force per unit area.
It's important to note that this formula assumes the force is evenly distributed over the entire area and that the surface is perpendicular to the force. In real-world scenarios, pressure may vary across a surface or be exerted at an angle, requiring more complex calculations or specialized formulas.
What are the different types of pressure?
In the context of pressure measurement, several types of pressure can be distinguished based on their reference points and the physical properties they represent. The main types of pressure include
- Absolute Pressure: Absolute pressure is measured relative to a perfect vacuum, where the pressure is zero. This is why absolute pressure is consistently positive. It represents the total pressure exerted by a fluid, including both atmospheric pressure and any additional pressure above it. Absolute pressure is commonly used in applications such as barometric pressure measurement, vacuum systems, and certain scientific and industrial processes.
- Gauge Pressure: Gauge pressure is measured relative to the local atmospheric pressure. It indicates the pressure above or below atmospheric pressure at a specific location. Gauge pressure readings can be positive (above atmospheric pressure) or negative (below atmospheric pressure). This type of pressure measurement is often used in industrial processes, automotive applications, and pressure measurement devices like tire pressure gauges.
- Differential Pressure: Differential pressure is the difference in pressure between two points or regions. It measures the change in pressure between two locations, rather than the absolute value. Differential pressure is widely used in flow measurement devices, such as orifice plates, venturi meters, and flowmeters, to calculate fluid flow rates by measuring the pressure drop across a constriction.
- Atmospheric Pressure: Atmospheric pressure is the pressure exerted by the Earth's atmosphere at a specific location. It represents the weight of the air column above that point. Atmospheric pressure varies with altitude and weather conditions. It is typically measured in units like millibars (mbar), hectopascals (hPa), or inches of mercury (inHg). Atmospheric pressure measurements are crucial in weather forecasting, aviation, and altitude calculations.
- Sealed or Enclosed Pressure: Sealed pressure refers to the pressure measured inside a sealed or closed system. It represents the pressure exerted by the contained fluid or gas, isolated from the atmospheric pressure. Sealed pressure is commonly monitored in pressurized vessels, pipelines, hydraulic systems, and other enclosed systems.
It's important to note that these different types of pressure are interrelated, and the choice of pressure measurement depends on the specific application and reference point of interest. Converting between different types of pressure can often be achieved by adding or subtracting the appropriate reference pressure (e.g., atmospheric pressure) from the measured value.
Absolute Pressure
- Range: The range of absolute pressure depends on the application and the pressure sensor used. It can range from near-zero values (vacuum) to several thousand or million pascals (Pa).
- Formula:
Pabs = Pgauge + Patm
where P abs is the absolute pressure, Pgauge is the gauge pressure, and Patm is the atmospheric pressure.
- Formula Example: If the gauge pressure is 500 Pa and the atmospheric pressure is 100,000 Pa, the absolute pressure would be 100,500 Pa.
- Units: The SI unit for absolute pressure is the Pascal (Pa).
- Usage: Absolute pressure is used in applications such as barometric pressure measurements, vacuum systems, pressure measurements in enclosed spaces, and scientific and industrial processes.

Fig 4. Absolute zero pressure
Gauge Pressure
- Range: Gauge pressure typically covers a range of positive values above atmospheric pressure or negative values below atmospheric pressure. The range depends on the specific application and the pressure sensor used.
- Formula:
Pgauge = Pabs - Patm
where P gauge is the gauge pressure, Pabs is the absolute pressure, and Patm is the atmospheric pressure.
- Formula Example: If the absolute pressure is 100,500 Pa and the atmospheric pressure is 100,000 Pa, the gauge pressure would be 500 Pa.
- Units: The SI unit for gauge pressure is pascal (Pa). Other units include psi, bar, or inches of the water column.
- Usage: Gauge pressure is commonly used in industrial processes, automotive applications, tire pressure measurement, pressure gauges, and pressure measurement devices.
Differential Pressure
- Range: The range of differential pressure varies widely depending on the specific application and the devices used. It can range from very low values (millipascals) to extremely high values (megapascals).
- Formula:
ΔP = P2 - P1
where ΔP is the differential pressure, P 2 is the pressure at the second point, and P1 is the pressure at the first point.
- Formula Example: If the pressure at the second point is 500 Pa and the pressure at the first point is 200 Pa, the differential pressure would be 300 Pa.
- Units: The SI unit for differential pressure is the pascal (Pa) or other units like psi, bar, or inches of the water column.
- Usage: Differential pressure is extensively used in flow measurement devices, such as orifice plates, venturi meters, flowmeters, and pressure drop calculations across filters or heat exchangers.
Atmospheric Pressure
- Range: The range of atmospheric pressure varies with altitude and weather conditions. It is typically around 100,000 pascals (Pa) at sea level but decreases with increasing altitude.
- Units: Atmospheric pressure is often measured in units like millibars (mb), hectopascals (hPa), or inches of mercury (inHg).
- Usage: Atmospheric pressure is crucial in weather forecasting, aviation, altimetry, barometric measurements, and understanding the behavior of gases at different elevations.
Sealed or Enclosed Pressure
- Range: The range of sealed or enclosed pressure depends on the specific application and the pressure limits of the system or equipment involved.
- Units: Sealed pressure is typically measured in pascals (Pa) or other units like psi, bar, or inches of water column.
- Usage: Sealed pressure measurements are employed in various applications, including pressurized vessels, pipelines, hydraulic systems, pneumatic systems, and other enclosed systems where monitoring the pressure within the system is important for operational safety and efficiency.
It's important to note that the formulas provided here are simplified representations, and actual pressure measurements may involve additional factors like calibration, temperature compensation, and sensor characteristics. The units mentioned are commonly used, but different industries or regions may have their preferred units of measurement.

Fig 5. Dynamic and static pressure
Comparing table
Here's a comparison table summarizing the different types of pressure:
| Pressure Type | Range | Formula | Units | Usage |
| Absolute Pressure | Near-zero to high | P abs = Pgauge + Patm | Pascal (Pa) | Barometric pressure, vacuum systems, scientific and industrial processes |
| Gauge Pressure | Positive or negative | P gauge = Pabs - Patm | Pascal (Pa), psi, bar, inches of water column | Industrial processes, automotive applications, tire pressure measurement, pressure gauges |
| Differential Pressure | Varies widely | ΔP = P 2 - P1 | Pascal (Pa), psi, bar, inches of water column | Flow measurement devices, pressure drop calculations |
| Atmospheric Pressure | Varies with altitude | - | Millibars (mb), hectopascals (hPa), inches of mercury (inHg) | Weather forecasting, aviation, altimetry, barometric measurements |
| Sealed Pressure | Depends on the system | - | Pascal (Pa), psi, bar, inches of water column | Pressurized vessels, pipelines, hydraulic systems, pneumatic systems, enclosed systems |
Please note that the table provides a general overview, and specific ranges and units may vary based on different applications and regional conventions.
What is the concept of pressure?
The concept of pressure is based on the fundamental principles of force and area. Here are the key concepts related to pressure:
- Force: Force is a physical quantity that describes the push or pull applied to an object. It is measured in newtons (N) and is responsible for causing a change in the motion or shape of an object. In the context of pressure, force refers to the amount of "push" or "squeeze" exerted on a surface.
- Area: Area refers to the extent or size of a surface. It is measured in square meters (m²). The area over which a force is applied is a crucial factor in determining pressure. A larger area spreads out the force, resulting in lower pressure, while a smaller area concentrates the force, leading to higher pressure.
- Pressure: Pressure is a measure of the amount of force applied per unit area. It quantifies the intensity or strength of the force exerted on a surface. Pressure is calculated by dividing the force perpendicular to the surface by the area over which the force is distributed. It is expressed in pascals (Pa) in the SI system.
- Pascal's Law: Pascal's law, also known as the principle of transmission of fluid pressure, states that a change in pressure applied to an enclosed fluid is transmitted equally in all directions. This principle forms the basis for hydraulic systems and allows for the amplification and control of forces through fluid pressure.
- Pressure Gradient: The concept of pressure gradient refers to the change in pressure over a given distance or in a particular direction. Pressure gradients are encountered in fluid dynamics and play a significant role in understanding fluid flow and determining the direction and speed of fluid motion.
- Pressure Measurement: Pressure can be measured using various devices called pressure sensors or transducers. These devices convert the physical force exerted on them into an electrical signal, which can be quantified and displayed. Pressure measurements are crucial for monitoring and controlling industrial processes, ensuring safety in various systems, and understanding fluid behavior.
Understanding the concept of pressure is essential in various fields, including physics, engineering, meteorology, fluid dynamics, and many branches of science and technology. It helps in designing structures and systems that can withstand pressure, calculating mechanical stresses, analyzing fluid flow, and ensuring the efficient operation of different processes.

Fig 6. Pressure formula
What are the properties of pressure?
Pressure possesses several important properties that help define its characteristics and behavior. Here are the key properties of pressure:
- Magnitude: Pressure has a quantitative nature and is expressed as a numerical value. The magnitude of pressure indicates the intensity or strength of the force exerted on a surface. It can range from low values (such as in a vacuum) to extremely high values (such as in hydraulic systems or industrial processes).
- Direction: Pressure is a vector quantity, meaning it has both magnitude and direction. The direction of pressure is always perpendicular to the surface on which it acts. The force applied to the surface is distributed evenly in all directions, resulting in pressure being exerted uniformly perpendicular to the surface.
- Units of Measurement: Pressure is measured using various units, depending on the context and the system of measurement being used. The SI unit for pressure is the pascal (Pa), which is equivalent to one newton per square meter (N/m²). Other common units include pounds per square inch (psi), atmospheres (atm), and bar.
- Relation to Force and Area: Pressure is fundamentally related to force and area. It is calculated by dividing the force exerted perpendicular to a surface by the area over which the force is distributed. The pressure increases with an increase in force or a decrease in area, and vice versa, provided the force is applied perpendicularly to the surface.
- Transmission in Fluids: Pressure in fluids follows Pascal's law, which states that pressure changes in an enclosed fluid are transmitted equally in all directions. This property allows pressure to be transmitted through fluids, leading to applications such as hydraulic systems and hydraulic pressure amplification.
- Pressure Gradient: A pressure gradient refers to the change in pressure over a given distance or in a particular direction. It describes the rate at which pressure changes spatially or along a flow path. Pressure gradients are encountered in fluid dynamics and are responsible for fluid motion and flow behavior.
- Atmospheric Pressure: Atmospheric pressure is the pressure exerted by the Earth's atmosphere at a specific location. It varies with altitude and weather conditions. Atmospheric pressure decreases with increasing altitude due to the decreasing weight of the air column above. Understanding atmospheric pressure is crucial in meteorology, aviation, and barometric measurements.
These properties of pressure help define and characterize its behavior, allowing for its measurement, control, and application in various fields and industries.
What does pressure depend on?
Pressure depends on several factors, including:
- Force: Pressure is directly proportional to the force applied. As the force increases, the pressure exerted on a surface or within a fluid also increases. Similarly, reducing the force decreases the pressure.
- Area: Pressure is inversely proportional to the area over which the force is distributed. When the same force is applied over a larger area, the pressure decreases. Conversely, applying the same force over a smaller area results in higher pressure.
- Fluid Density: In the case of fluid pressure, the density of the fluid plays a role. For a given force and area, the pressure exerted by a fluid increases with higher fluid density. Denser fluids contain more particles per unit volume, leading to greater pressure exerted on a surface.
- Depth/Height: In fluid systems, pressure is influenced by the depth or height of the fluid column. This relationship is known as hydrostatic pressure. The pressure at a certain depth increases with the weight of the fluid above that point. The deeper the fluid or the higher the fluid column, the greater the pressure.
- Gravity: Gravity also affects pressure, especially in fluid systems. The weight of the fluid column creates pressure, and gravity provides the force for this weight. The greater the gravitational force acting on a fluid, the higher the pressure.
- Temperature: In some cases, temperature can have an impact on pressure. For ideal gases, pressure is directly proportional to temperature according to the ideal gas law (PV = NRT). However, this relationship may not hold for all substances or systems.
It's important to note that these factors interact with each other and may vary depending on the specific context or system being considered. Understanding these dependencies is crucial for accurately analyzing and predicting pressure in various scenarios.
What is pressure in different states of matter?
Pressure exists in different states of matter—solid, liquid, and gas—but its characteristics can vary based on the properties and behavior of each state.
- Solid: In solids, pressure refers to the force applied to a solid object divided by the area over which the force is distributed. Pressure in solids arises from the compression or deformation of the material under external forces. It is often measured in terms of mechanical stress, which is the force per unit area acting on the material. The pressure distribution in solids can vary depending on the shape, structure, and mechanical properties of the material.
- Liquid: In liquids, pressure refers to the force exerted by the liquid on the walls of a container or objects immersed in the liquid. Liquid pressure is a result of the weight of the liquid above the given point, as well as the forces exerted by the molecules colliding with the container or objects. In a liquid, pressure is transmitted equally in all directions due to the fluid's ability to flow and conform to the shape of the container. This property is described by Pascal's law.
- Gas: In gases, pressure refers to the force exerted by gas molecules on the walls of a container. Gas pressure arises from the random motion and collisions of gas molecules with the container or other objects. Gas pressure is affected by factors such as temperature, volume, and the number of gas molecules present. It is typically measured using instruments like pressure gauges or manometers.
In all three states of matter, pressure is a measure of the force per unit area and is important for understanding the behavior and properties of the material or substance. Pressure plays a role in various applications, ranging from structural analysis of solids to the operation of hydraulic systems, weather prediction based on atmospheric pressure, and control of gas systems in industrial processes.

Fig 7. Pressure definition
Absolute, Gauge, and Differential pressure measurements
Absolute Pressure
- Definition: Absolute pressure is the total pressure exerted by a fluid, including both the pressure above atmospheric pressure and any additional pressure below atmospheric pressure.
- Reference Point: Absolute pressure is measured concerning a perfect vacuum, where the pressure is considered zero.
- Measurement Range: Absolute pressure can range from near-zero values (vacuum) to high positive values depending on the application and the pressure range of the measurement device.
- Formula: P_abs = P_gauge + P_atm, where P_abs is the absolute pressure, P_gauge is the gauge pressure, and P_atm is the atmospheric pressure.
- Example: If the gauge pressure is 500 Pa and the atmospheric pressure is 100,000 Pa, the absolute pressure would be 100,500 Pa.
- Units: The SI unit for absolute pressure is the Pascal (Pa).
Gauge Pressure
- Definition: Gauge pressure is the pressure measured relative to the local atmospheric pressure.
- Reference Point: Gauge pressure is measured concerning atmospheric pressure, which is typically considered as zero or a reference point.
- Measurement Range: Gauge pressure can have both positive and negative values. Positive values indicate pressure above atmospheric pressure, while negative values indicate pressure below atmospheric pressure.
- Formula: P_gauge = P_abs - P_atm, where P_gauge is the gauge pressure, P_abs is the absolute pressure, and P_atm is the atmospheric pressure.
- Example: If the absolute pressure is 100,500 Pa and the atmospheric pressure is 100,000 Pa, the gauge pressure would be 500 Pa.
- Units: The SI unit for gauge pressure is the pascal (Pa), but it is also commonly expressed in units like psi, bar, or inches of the water column.
Differential Pressure
- Definition: Differential pressure is the difference in pressure between two points or regions.
- Reference Points: Differential pressure is not referenced to atmospheric pressure but rather focuses on the pressure difference between two specific locations.
- Measurement Range: Differential pressure can vary widely depending on the specific application and the devices used.
- Formula: ΔP = P2 - P1, where ΔP is the differential pressure, P2 is the pressure at the second point, and P1 is the pressure at the first point.
- Example: If the pressure at the second point is 500 Pa and the pressure at the first point is 200 Pa, the differential pressure would be 300 Pa.
- Units: The SI unit for differential pressure is the pascal (Pa), but it can also be expressed in units like psi, bar, or inches of the water column.
While absolute pressure and gauge pressure are absolute values, differential pressure represents the change or difference in pressure between two points or regions.

Fig 8. Gas pressure
What is the difference between absolute, gauge, and differential pressure measurements?
The differences between absolute, gauge, and differential pressure measurements lie in their reference points, the quantities they represent, and their applications. Here's a breakdown of the key distinctions:
Reference Point
- Absolute Pressure: Absolute pressure is measured relative to a perfect vacuum, where the pressure is considered zero. It represents the total pressure exerted by a fluid, including both the pressure above atmospheric pressure and any additional pressure below atmospheric pressure.
- Gauge Pressure: Gauge pressure is measured relative to the local atmospheric pressure. It indicates the pressure above or below atmospheric pressure at a specific location. Atmospheric pressure is typically considered as the reference point or zero in gauge pressure measurements.
- Differential Pressure: Differential pressure is not referenced to a specific pressure point or atmospheric pressure. It focuses on the difference in pressure between two specific points or regions.
Quantity Represented
- Absolute Pressure: Absolute pressure represents the total pressure, including both positive and negative pressures concerning a perfect vacuum.
- Gauge Pressure: Gauge pressure represents the pressure above or below atmospheric pressure at a specific location, indicating only the positive or negative pressure relative to atmospheric pressure.
- Differential Pressure: Differential pressure represents the difference in pressure between two points or regions, regardless of the reference pressure.
Measurement Range
- Absolute Pressure: The range of absolute pressure can span from near-zero values (vacuum) to high positive values, depending on the application and the measurement device.
- Gauge Pressure: Gauge pressure can have both positive and negative values. Positive values indicate pressure above atmospheric pressure, while negative values indicate pressure below atmospheric pressure.
- Differential Pressure: The measurement range of differential pressure varies widely based on the specific application and the devices used.
Formula and Calculation
- Absolute Pressure: The absolute pressure can be calculated by adding the gauge pressure to the atmospheric pressure: P_abs = P_gauge + P_atm.
- Gauge Pressure: Gauge pressure can be calculated by subtracting the atmospheric pressure from the absolute pressure: P_gauge = P_abs - P_atm.
- Differential Pressure: Differential pressure is calculated by subtracting the pressure at the first point from the pressure at the second point: ΔP = P2 - P1.
Units
- Absolute Pressure: The SI unit for absolute pressure is the pascal (Pa). Other common units include psi, bar, or inches of mercury (inHg).
- Gauge Pressure: The SI unit for gauge pressure is the pascal (Pa), but it is also commonly expressed in units like psi, bar, or inches of water column.
- Differential Pressure: The SI unit for differential pressure is the pascal (Pa), but it can also be expressed in units like psi, bar, or inches of water column.
Applications
- Absolute Pressure: Absolute pressure is used in applications such as barometric pressure measurements, vacuum systems, pressure measurements in enclosed spaces, and scientific and industrial processes.
- Gauge Pressure: Gauge pressure finds applications in industrial processes, automotive systems, tire pressure measurement, pressure gauges, and pressure measurement devices.
- Differential Pressure: Differential pressure is extensively used in flow measurement devices, pressure drop calculations, filtration systems, and various industrial and scientific processes that involve pressure differentials.
In summary, the key differences between absolute, gauge, and differential pressure measurements lie in their reference points, the quantities they represent, the calculation formulas, and their specific applications.
Here's a comparison table summarizing the differences between absolute, gauge, and differential pressure measurements:
| Aspect | Absolute Pressure | Gauge Pressure | Differential Pressure |
| Reference Point | Perfect vacuum (zero pressure) | Local atmospheric pressure | No specific reference point |
| Quantity Represented | Total pressure (positive and negative) | Pressure above or below atmospheric pressure | Difference in pressure between two points |
| Measurement Range | Near-zero to high positive/negative values | Positive and negative values | Varies widely based on application |
| Formula | P abs = Pgauge + Patm | P gauge = Pabs - Patm | ΔP = P 2 - P1 |
| Units | Pascal (Pa), psi, bar, inches of mercury | Pascal (Pa), psi, bar, inches of water column | Pascal (Pa), psi, bar, inches of water column |
| Applications | Barometric pressure, vacuum systems, | Industrial processes, automotive systems, | Flow measurement, pressure drop calculations, |
| pressure measurement in enclosed spaces | tire pressure measurement, pressure gauges | filtration systems, industrial processes |
Please note that while this table provides a general overview of the differences, specific applications, and measurements may have variations and specific requirements.
Conclusion
In conclusion, pressure is a fundamental concept in physics and plays a crucial role in various fields and industries. It represents the force applied per unit area and can be measured in different ways depending on the application.
Absolute pressure is the total pressure exerted by a fluid, including both positive and negative pressures, and is measured relative to a perfect vacuum. Gauge pressure is measured relative to the local atmospheric pressure, indicating the pressure above or below atmospheric pressure at a specific location. Differential pressure represents the difference in pressure between two points or regions, without referencing a specific pressure point.
The properties and behavior of pressure can vary depending on the state of matter, whether it be a solid, liquid, or gas. Pressure depends on factors such as force, area, fluid density, depth or height, and gravity. Understanding these factors is important for accurate pressure measurement and control in various applications.
Absolute, gauge, and differential pressure measurements have distinct reference points, quantities represented, measurement ranges, formulas, units, and applications. Absolute pressure is used in barometric measurements, vacuum systems, and scientific processes. Gauge pressure finds applications in industrial processes, automotive systems, and pressure measurement devices. Differential pressure is essential for flow measurement, pressure drop calculations, and various industrial processes.
By comprehending the differences and properties of absolute, gauge, and differential pressure measurements, one can effectively analyze and interpret pressure in different scenarios and industries.
To recap
Q: How is pressure related to temperature?
A: In gases, pressure is directly proportional to temperature according to the ideal gas law (PV = NRT), where P represents pressure, V represents volume, n represents the number of moles of gas, R is the gas constant, and T represents temperature. As temperature increases, the gas molecules gain more kinetic energy, resulting in increased molecular collisions with the container walls and thus higher pressure.
Q: What is the difference between pressure and stress?
A: Pressure and stress are related concepts but have different applications. Pressure is the force applied per unit area and is typically used to describe forces exerted on surfaces or fluids. Stress, on the other hand, refers to the internal forces within a solid material and is used to describe the response to external forces applied to a solid object. Stress is often expressed in terms of mechanical stress, which is the force per unit area acting on a material.
Q: Can pressure be negative?
A: Yes, pressure can be negative. Negative pressure indicates a pressure below the reference point, which is typically atmospheric pressure. For example, in a vacuum system, the pressure can be expressed as a negative value compared to atmospheric pressure.
Q: What instruments are used to measure pressure?
A: Pressure can be measured using various instruments called pressure sensors or transducers. Common types of pressure measurement devices include bourdon tube gauges, pressure transducers, pressure transmitters, manometers, and pressure gauges. The choice of instrument depends on the specific application, measurement range, accuracy requirements, and environmental conditions.
Q: How is pressure converted between different units?
A: Pressure can be converted between different units using conversion factors. For example, to convert pascals (Pa) to pounds per square inch (psi), the conversion factor is approximately 0.00014504. So, to convert Pa to psi, you would multiply the value in Pa by 0.00014504. Similarly, conversion factors exist for other units such as bar, atmospheres, and inches of water column. It's important to use the appropriate conversion factor and ensure consistency in unit conversions.
Q: What is the typical atmospheric pressure at sea level?
A: At sea level, the typical atmospheric pressure is approximately 101,325 pascals (Pa) or 1 atmosphere (atm). This is often used as a reference point for gauge pressure measurements.
Q: What are the safety considerations related to high pressure systems?
A: High-pressure systems require careful safety considerations. It is important to ensure the integrity and strength of equipment, use appropriate materials and fittings designed for high pressure, regularly inspect and maintain pressure vessels, follow proper installation and operation procedures, and implement safety measures to prevent over-pressurization or sudden pressure releases. Training, risk assessments, and compliance with relevant regulations are essential for safe operation of high-pressure systems.
References
https://www.tec-science.com/mechanics/gases-and-liquids/pressure/
https://greenishco.com/PressureDefinition
https://www.britannica.com/science/pressure
https://www.khanacademy.org/science/physics/fluids/density-and-pressure/a/pressure-article
https://ur.javamem.com/pictures/science-pressure-examples
https://thirdspacelearning.com/gcse-maths/ratio-and-proportion/pressure-formula/
Recent Posts
-
Booster Pump Troubleshooting and Maintenance: How to Fix and Prevent Common Issues
1. Introduction Imagine turning on your faucet only to be greeted with a weak trickle of water when …22nd Apr 2025 -
Energy-Efficient Booster Pumps: Selection and Tips for Maximizing Performance
1. Introduction Imagine never having to deal with fluctuating water pressure, noisy pumps, or skyroc …19th Apr 2025 -
Booster Pumps for Sustainable Water Systems: Irrigation and Rainwater Harvesting Solutions
1. Introduction Water scarcity is no longer a distant threat—it’s a reality affecting millions …16th Apr 2025




